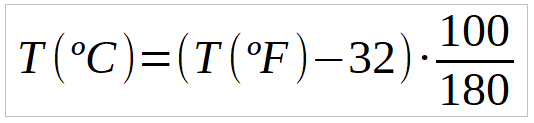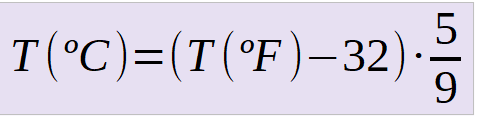Temperature and thermal energy
Matter is made up of particles that are in continuous motion. Thermal energy is the form of energy due to the agitation of the particles that make up matter at the molecular level.
As the particles move faster and collide with each other, the temperature of the object increases, which in turn increases its thermal energy.
Temperature is a scalar quantity that indicates the amount of kinetic energy of the particles of a body. The greater the speed of the particles, the higher the temperature and vice versa. In the following video you can see how water particles would move at 10 ºC and at 80 ºC. Observe the difference in speed and in the space occupied by the same amount of particles at each temperature.
Video based in PhET Interactive Simulations, University of Colorado Boulder, PHET, CC-BY-04
Music: Mourning Dove, Zachariah Hickman, Youtube studio
At constant pressure, as the temperature increases, the speed at which the particles move and the space they occupy increases. Therefore, the volume occupied by the substance will increase and its density will decrease .
The increase in volume of a body as its temperature increases is called expansion, and is the basis for the operation of traditional thermometers.